Essential oils are used in various consumer goods such as detergents, soaps, toilet products, cosmetics, pharmaceuticals, and perfumes. The world’s production and consumption of essential oils are increasing very fast due to the commercialization of natural health care products. Therefore, production technology is a critical element in improving the overall yield and quality of these oils.
This article will briefly discuss methods for essential oil extraction from plant materials like flowers, seeds, leaves, or roots, which gives a significant contribution to improvement in yield and quality of the extracted product.
Have you ever wondered how essential oils are made? First, let us clarify that Essential Oils are not manufactured but rather extracted from plant materials. Extracts are used to isolate the active botanical constituents of a plant that serve as its “life force.” They are a plant’s liquefied form. As a result, they efficiently expedite the plant’s beneficial compounds into the bloodstream compared to merely eating the plant.
Hydro-Distillation
The essential oils industry has evolved to differentiate three hydro-distillation methods: water distillation, water, and steam distillation, and direct steam distillation. The most significant distinction is in the ways of treating the material.
The hydro-distillation process is the most common and simplest of all extractions. The heat builds up gradually in this method from low temperatures at first, then reaches boiling point later on. This gradual increase in temperature causes water vapor to be released from plant material suspended in near-boiling water inside the still pot without harming or changing it chemically. When steam rises into the cooling coil, it has cooled down and condensed back to liquid form (pure H20). These droplets trickle out of a spout cap positioned over an opening onto waiting receptacles. They are collected for use or further processing.
1. Water Distillation
The plant material is fully submerged in water in this process, which is then boiled by applying heat by direct fire, steam jacket, closed steam jacket, sealed steam coil, or open steam coil. The key feature of this method is the direct interaction between boiling water and plant material.

Source: New Directions Aromatics
When delicate flowers such as roses and orange blossoms were exposed to steam during the distillation process, they clumped together. Submerging delicate plant material in pure boiling water is the most efficient process. Water helps prevent overheating of the extracted oil. Furthermore, the concentrated liquids cool and segregate. The remaining water, which may be fragrant in some cases, is referred to by various terms, including hydrolase, hydrosol, herbal water, essential water, floral water, or herbal distillate.
Water is boiled by applying heat, and it extracts the essential oils. This process requires less plant material than steam distillation does, so there’s a faster extraction time. The significant advantage of this method is that water doesn’t contain any impurities or residues like a solvent, which means that using distilled water will create purer oil with no contaminants in the final product.
Another benefit of water distillers is that they’re smaller and weaker when compared to other methods for extracting essential oils from plants (which makes them cheaper). One disadvantage of this process is that some compounds may not be soluble in pure enough water at high enough temperatures because the boiling point for different substances varies.
Advantages of Water Distillation
One distinct benefit of water distillation is that it enables finely powdered material or plant pieces. Additionally, the practical advantages of water distillation include the stills’ low cost, ease of construction, and suitability for field operation. These are also commonly used in many countries with portable equipment.
Disadvantages of Water Distillation
The primary disadvantage of water distillation is the inability to obtain a complete extraction. Additionally, certain esters are partially hydrolyzed, and sensitive substances such as aldehydes polymerize, resulting in the mixing of high-quality oil with low-quality oil. Water distillation requires more stills, additional space, and additional fuel than alcohol distillation. It is a more time-consuming process than water and steam distillation or direct steam distillation.
This is why water distillation is only used in situations where the plant component itself cannot be distilled by steam distillation or water and steam.
2. Water and Steam Distillation
In general, the equipment used in water and steam distillation is comparable to water distillation. Nevertheless, a perforated grid supports the plant material above the boiling water.
After distillers have developed a few batches of oil through water distillation, they notice that the oil’s reliability is poor due to its still notes. As a consequence, several changes are implemented. First, a perforated grid or plate is used to elevate the plant material above the water. This process decreases the potential of the still while improving the oil quality.
Advantages of Water and Steam Distillation over Water Distillation
Hydrolysis and polymerization are less likely to occur with the volatile oil’s components. Additionally, the oil produced by steam and water distillation is of higher quality than oil produced by water distillation. Furthermore, distillation is a more energy-efficient process.
Disadvantages of Water and Steam Distillation
Due to the low pressure created by rising steam, high-boiling oils require greater moisture to vaporize, requiring longer distillation hours.
Compared with water distillation, the process of water and steam distillation process is more complex. The yield from a given quantity of plant material may be less than that obtained by water alone in some cases. And certain oils decompose or polymerize when exposed to air for any length of time hence make unsuitable candidates for this type of extraction process.
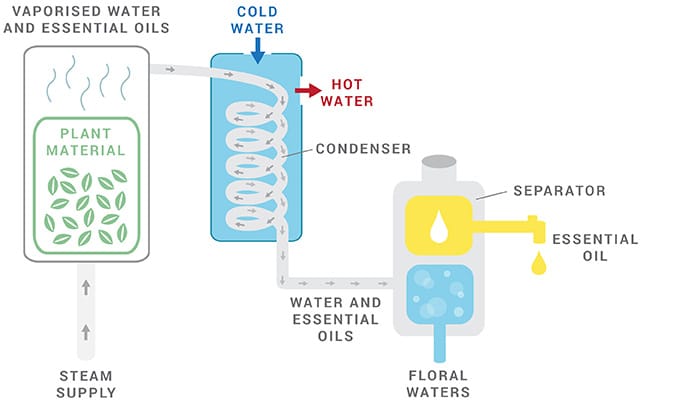
3. Direct Steam Distillation
Direct steam distillation is the most frequently used method for producing essential oils.
Source: New Directions Aromatics
The process of direct steam distillation is relatively simple and can be done on a small scale. First, heating the plant material in water to release volatile compounds is then collected using cold air or iced water to condense back into liquid form.
Steam distillation is the most widely accepted method for the large-scale production of essential oils. This is standard practice throughout the flavor and fragrance supply industry.
One key advantage of this process is that it does not require any other chemicals for extraction, such as solvents like ethers or butane. The main disadvantage with direct steam distillation is that some components may get distilled off at very low levels due to their volatility point being above 100 ° C (212 ° F). In contrast, others will remain behind because they have higher boiling points than 100 °C. This means you might only extract part of essential oil that’s available from the plant materials.
Advantages of Direct Steam Distillation
We can easily control the amount of steam. There is no thermal decomposition of oil constituents and which is a significant advantage of direct steam distillation.
This distillation method, which is superior to the other two techniques, is the most widely accepted process for large-scale oil production.
The Disadvantage of Direct Steam Distillation
This method requires significantly more capital investment than the other two processes.
Expression
4. Expression (Cold-pressing)
Expression is also known as cold-pressing or scarification and is used for citrus peels in particular. In the past, expression is done manually by soaking citrus peels in warm water first, then presses them with a sponge over an open container to separate the flavor and fragrance. This process has been replaced by extractors that do most of the work for you.

Source: New Directions Aromatics
The most commonly used expression process is pelatrice or sfumatrice, which produces bergamot oil and lemon oils from Italy’s production area.
Hand pressing is inefficient because it is a time-consuming process; for example, a single person can produce only 2-4 lbs of oil per day using one of these hand methods. As a result, several devices have been built over the years to either crush the peel of citrus fruit or crush the whole fruit. The resulting liquid is centrifuged, separating it into citrus juice and essential oils.
Solvent Extraction
Petroleum solvents such as petroleum ether, hexane, or toluene; alcohol solvents such as methanol or ethanol; or carbon dioxide are used in this process. The solvent is absorbed by the botanical as it is applied, allowing the aromatic compounds to be released.
Source: New Directions Aromatics
It is ideal for plant materials that produce little essential oil and are mainly resinous or delicate aromatics that cannot endure the pressure and distress of steam distillation. This process also yields a more delicate scent than any other method of distillation.
Solvent extraction is often used to produce oils of high value, which would otherwise be too expensive or thermally unstable for distillation. These include jasmine (which is a resinous material), tuberose, and hyacinth. It can also extract fragrant plant materials such as hops from the brewing industry.
The following methods are included in Solvent Extraction: CO₂ Extraction (Carbon Dioxide), Enfleurage, Petroleum, and Ethanol Solvent.
5. CO₂ Extraction
CO₂ extraction, also known as Supercritical Carbon Dioxide Extraction, has been steadily gaining popularity as a more natural method to extract the oil from plants. This process can produce higher quality oils that have not been altered by applying high heat, unlike in the steam distillation process, where none of the oil constituents are damaged by heat.

Source: New Directions Aromatics
The distinction between conventional distillation and carbon dioxide distillation is that CO₂ is used as a solvent in the latter process rather than heated water or steam. The temperature of the carbon dioxide is raised to about 92 °F (33 °C) under pressure. Carbon dioxide reaches a phase that is part liquid and part gas at this temperature. As a result, carbon dioxide is an ideal solvent for extracting pure essential oils because it does not chemically interfere with the extracted essential oil. Reduce the strain to extract the carbon dioxide solvent. As a result, the carbon dioxide returns to a gaseous state, leaving behind the pure essential oil.
The CO₂ extraction process occurs at temperatures ranging from 91 to 100 °F (35 to 38 °C). On the other hand, steam distillation occurs at temperatures ranging from 140 to 212 °F (60 to 100 °C).
CO₂ extracts are usually thicker than essential oil extracts. They often emit more of the natural fragrance of the herb, spice, or plant than a distilled essential oil. CO₂ extracts are said to contain more plant constituents than steam distillation extracts from the same plant.
6. Enfleurage
Enfleurage is an ancient form of essential oil extraction that makes use of fat. Despite the introduction of the modern method of extraction with volatile solvents, enfleurage is still used today. However, large-scale enfleurage is now only practiced in the Grasse region of France, with the possible exception of a few isolated cases in India, where the procedure has remained primitive.
The concepts of enfleurage are straightforward. Certain flowers (for example, tuberose and jasmine) continue to grow and emit perfume even after being picked. Every jasmine and tuberose herb, in a way, resembles a small factory constantly emitting minute amounts of perfume. Fat has a high absorption capacity and readily absorbs the fragrance released when in contact with fragrant flowers. Enfleurage is the systematic application of this theory on a broad scale. Throughout the harvest time, which lasts eight to ten weeks, batches of freshly picked flowers are sprayed over the surface of a specially prepared fat base (corps), allowed to sit (for 24 hours in the case of jasmine and longer in the case of tuberose), and then replaced by fresh flowers. The fat, which is not renewed during the process, is saturated with flower oil at the end of the harvest. Following that, the oil is separated from the fat using alcohol and then isolated.
By the end of this procedure, vegetable or animal fat has been saturated with the fragrance compounds of the herb. At room temperature, the fats used are odorless and robust. Enfleurage may be performed “hot” or “cold.” In both cases, the saturated fat with fragrance is referred to as “enfleurage pomade.”
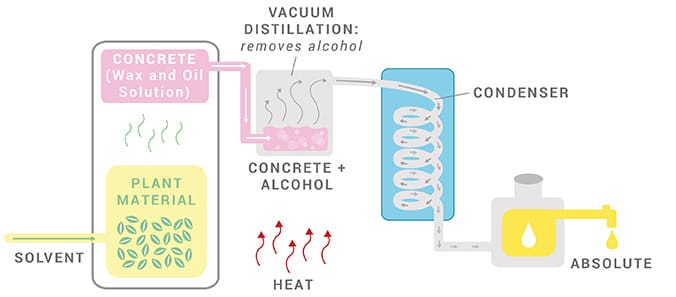
7. Petroleum and Ethanol Solvent
Petroleum Solvent: Until a few years ago, this method was used to remove almost all absolutes, essential oils, and even carrier oils. However, since petroleum solvent residues such as hexane or benzene can remain in the aromatic oil, we do not recommend solvent extraction for essential oils used in aromatherapy. These residues are also capable of causing allergic reactions and skin irritation.
Ethanol Solvent: Though petroleum solvents may be harmful, ethanol (drinking alcohol of food grade) is a relatively benign solvent. Ethanol is used to steep the plant material. The alcohol is then evaporated, leaving behind a highly concentrated oil known as an absolute. Occasionally, an absolute, such as vanilla, is listed as an essential oil, but absolutes are not technically essential oils.
The majority of modern essential oil production in the perfume industry is obtained by extraction using volatile solvents such as petroleum ether and hexane. The primary benefit of solvent extraction over distillation is that manufacturers of essential oils can maintain a constant temperature of 122 °F (typically 50 °C) during the process. As a result, extracted oils have a more natural aroma that distilled oils cannot balance. In addition, the high temperature may have chemically altered. This property is critical to the perfume industry, but the proven distillation method is less efficient than the extraction process.
Get free guides & sources to help grow your aromatherapy business.
No charge. Unsubscribe anytime.





